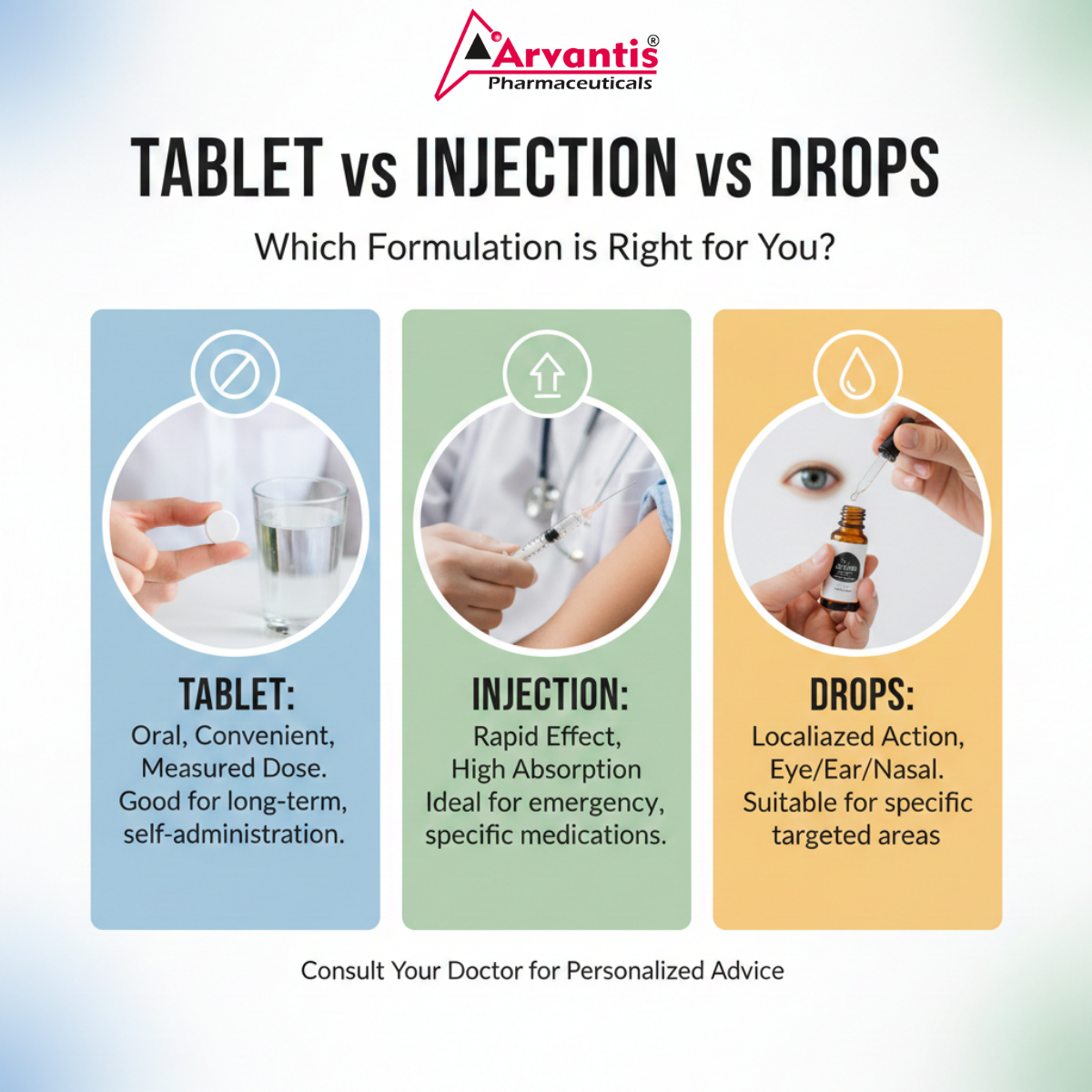
Tablet vs Injection vs Drops Which Formulation is Right for You
Introduction
-
Why formulation matters: delivery, patient compliance, stability
-
Who this post is for (hospitals, distributors, clients)
1. Tablet / Capsule
-
Definition & types (immediate release, sustained release)
-
Advantages (convenience, cost, shelf life)
-
Disadvantages & limitations
-
Typical use cases (chronic diseases, mass distribution)
2. Injection / Injectable
-
Definition (IM, IV, subcutaneous)
-
Advantages (fast onset, direct delivery)
-
Challenges: sterility, cold chain, cost
-
Use cases (emergency, vaccines, biologics)
3. Drops / Liquid Formulation
-
Definition (ophthalmic, ear, nasal)
-
Pros: ease of use, local effect
-
Cons: stability, preservatives, precision dosing
-
Scenarios where drops are preferred
4. How to Choose the Right Formulation
-
Patient demographics (age, compliance)
-
Cost vs benefit tradeoff
-
Storage / logistics constraints
-
Regulatory / licensing considerations
5. Our Expertise & Quality Assurance
-
How Your Company manufactures all types reliably
-
QC processes, GMP, certification
Conclusion / Call to Action
-
Summary of key points
-
Suggest contacting you for consultation or sampling




